VirotherapyDestroyCancerCell.jpg

Image from RIGVIR® video
Cancer virotherapy
Cancer virotherapy is one of the most promising modern cancer therapy methods. Virotherapy is an effective cancer treatment that uses oncotropic and oncolytic viruses with the ability to find and destroy malignant cells (cancer) in the body. Virotherapy is gentle and safe treatment; it improves survival and quality of patient's life. Also, it has the ability to harness patient’s own immune system to help fight cancer.
The purpose of oncolytic virotherapy is no longer a purely academic exercise. At the moment many pharmaceutical companies throughout the world are trying to develop virotherapy medicines, and, at the same time, there are many clinical trials in various phases.
The first registered virus that is known to have oncolytic efficacy is RIGVIR® virus (Picornaviridae genera, Enterovirus genus, ECHO group, type7, Rigvir). It is a live, adapted, not genetically modified, non-pathogenic virus with oncotropic and oncolytic properties.
Virotherapy's history
Dock G. reported in 1904 a case of leukemia in a woman who had a complete but temporary remission after an upper respiratory infection, presumed to be influenza.
Other authors reported similar observations of spontaneous remissions of leukemias and lymphomas after infections such as chicken pox, measles or hepatitis.
With the advent of human cell culture in the 1940s and 1950s, molecular biology in the 1970s, the ability to study genetic material outside body became feasible. The concept of genetic therapy was a logic and a new field arose addressing diseases with identifiable genetic abnormalities by alteration of existing genetic material or introduction of new genetic material.
Latvian virotherapy's story
In 1960 the scientists detected that the human intestinal viruses, obtained from young children, can destroy the human tumors which are engrafted to hamsters.
In 1965 the laboratory of cancer virotherapy was organized to investigate this phenomenon. Under the guidance of Prof. Aina Muceniece more than 70 different types of the intestinal viruses were estimated. One of them was named – RIGVIR (Riga virus).
The adaptation of RIGVIR (specifically) for the skin melanoma tissues was started because this tumor is not sensitive to radiation and chemical therapy. The adaptation of the virus for the melanoma was successful and the therapeutic effect of RIGVIR adapted in melanoma was detected.
In 1985 the positive conclusion from the Scientific Council of Soviet Cancer Centre (Moscow) was received for the extensive clinical trials of using RIGVIR in the treatment of melanoma patients.
In 1987 the clinical trials were done in the Oncology Centre in Moscow with the permission of the Pharmacology Committee of USSR where the safety of RIGVIR for even very difficult, untreatable patients was approved.
How does RIGVIR® work?
RIGVIR® is oncolytic virus. It finds and infects cancer cells – this process is called oncotropism. Then a live virus replicates in cancer cells and destroys them – this process is called oncolysis. Both processes are selective for malignant cells but healthy cells are minimally, if at all affected and also it does not cause adverse events.
Mechanism of Action
rigvir-mechanism.jpg
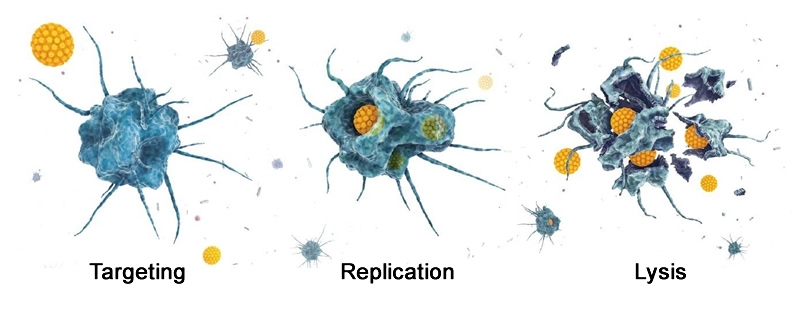
Image courtesy of RIGVIR®
RIGVIR® targets cancer cells, replicates inside them, and then destroys the cancer cells.
Indications
The primary indication for this medicine is melanoma, but it also shows great effectiveness in the following types of cancers – stomach, colorectal, pancreatic, lung, prostate cancers and others, as well as, it is used for prevention of its recurrences, metastases and secondary immune deficiency. It also functions as an immunomodulator.
Virus properties: oncotropic and oncolytic, well-tolerated, high therapeutic index, improves quality of life, non-genetically modified, immunomodulating efficacy, ambulatory use, safe and effective.
Efficacy and Safety
A recent clinical retrospective study published in Melanoma Research (Oct. 2015, Vol. 25, pp. 421-426 ) indicates that melanoma patients would significantly benefit from prolonged survival with virotherapy treatment. The study revealed that the IIB - IIC stage melanoma patients treated with RIGVIR® were 4-6 times more likely to survive than those who, following the current guidelines, were only observed.
In a retrospective study published by Latvijas Ārsts the time to progression in stage II melanoma patients was measured. The results show that patients under observation have 6.7-fold higher risk of disease progression compared with those that were treated with RIGVIR®.
RIGVIR® demonstrates an outstanding safety profile. About 2,000 patients participated in various clinical studies, half in safety, half in efficacy studies. Since its approval and registration, no serious adverse events have been recorded. Side effects are short-term and do not require special therapy. The most common symptom is subfebrile temperature for 1-3 days.
Latest news
Oncolytic virotherapy is on the spotlight today because recent clinical trials and experience shows it as a very promising treatment, which was expected by patients and doctors a long time ago. Year 2015 has been a real a breakthrough for RIGVIR® because of its inclusion into national clinical guidelines for skin cancer and melanoma treatment, as well as approval in one more country: Georgia.
Future
It is predicted that there will be 23.6 million new cancer cases worldwide each year by 2030, if recent trends in incidence of major cancers and population growth are seen globally in the future. This is 68% more cases than in 2012 (International Agency for Research on Cancer).
Meanwhile, today’s well known drugs and therapies are unable to manage the situation in the field of oncology or increase the survival and quality of life of the patient suffering from cancer. It is becoming clear that current cancer treatment methods would be substituted with new and more effective solutions.
Please see the video accompanying this article for more information. To learn more about RIGVIR® go to the International Virotherapy Center website.
From Idea To Result - Rigvir, Latvian Virotherapy center
International Virotherapy Center
References
Latvijas Ārsts from the International Virotherapy Center


